Spray polyurethane foams (SPF) are gaining popularity in the construction industry and are a valuable product for insulating or sealing gaps and cracks in the building envelope. There are two main systems for the application of SPF: the one component system and the two component system. This article will attempt to explain the uses and chemistry behind each, as well as health implications of SPF use.
The One Component System
The one-component system contains all reactants in a pressurized can. This system is used mainly for air-sealing small gaps, cracks and holes in the building envelope, such as those around windows and doors, service penetrations, and small cavities that are too difficult to insulate otherwise.
The Two Component System
In the two-component system, reactants are kept in separate containers and mixed at the nozzle of a spray gun to begin the polymerization and blowing reactions. One container contains the isocyanates (A side), and the other contains the polyols and various additives (B side). Some manufacturers replace a small portion of the petroleum-derived chemicals in the B side with soy-derived chemicals to increase the renewability of their product. The two-component system is used to apply SPF on a large scale, such as insulating between trusses or studs in a wall. Two types of SPF, closed-cell and open-cell, may be used as insulation and each as its own benefits.
Types of Spray Foam
Closed-cell SPF has a medium density, high strength, and high rigidity. It acts as a vapor barrier when applied thicknesses of over 2” in climates up to 10.000 Heating Degree Days (HDD) and when interior relative humidities are up to 50% (Straube, 2009). It is an effective air barrier and has an R-value of over 6 per inch (American Chemistry Council, 2016).
Open-cell SPF has a low density, low strength, and low rigidity (American Chemistry Council, 2016) and has been shown to act as a vapor barrier in climates with less than 4,500 HDD when applied at full wall thickness (5.5”) (Straube, 2009), and an air barrier when applied at the same thickness. It has an R-value of about 3.5 per inch (American Chemistry Council, 2016).
Open-cell SPF has a low density, low strength, and low rigidity (American Chemistry Council, 2016) and has been shown to act as a vapor barrier in climates with less than 4,500 HDD when applied at full wall thickness (5.5”) (Straube, 2009), and an air barrier when applied at the same thickness. It has an R-value of about 3.5 per inch (American Chemistry Council, 2016).
When to Insulate With Spray Foam
Insulating wall or truss cavities with SPF is ideal during a renovation, but not during new home construction. The framing in new homes has a tendency to shrink, warp, and expand as it adjusts to the humidity levels of the home. During this process, sprayed foam may separate from framing members, detracting from its ability to act as an air or vapor barrier if this was its intended function. Framing members in older homes have already undergone this distortion process, and are much less likely to experience these issues than new homes.
Spray Foam Chemistry
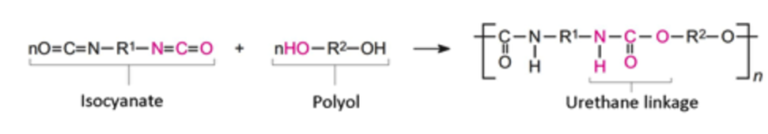
The main ingredients in SPF are isocyanate compounds (most commonly 4,4’-diphenylmethanediisocyanate and its isomers) and a polyol (Environmental Protection Agency, 2016). Additives such as flame retardants, catalysts, and blowing agents serve several functions (Environmental Protection Agency, 2016). Flame retardants reduce the flammability of the hardened polyurethane foam to meet building codes. Catalysts increase the rate of the polymerization reaction. The reaction between the isocyanates and the polyols generates heat, and this heat enables the blowing agents to expand and ‘bubble’, within the hardening polyurethane, creating the cells within the foam (Straube, 2009).
What does this reaction look like? The polyurethane foam is created when the cyanate group of an isocyanate molecule reacts with the hydroxyl group of a polyol, forming a urethane linkage between two isocyanate molecules (The University of York, 2013).
What does this reaction look like? The polyurethane foam is created when the cyanate group of an isocyanate molecule reacts with the hydroxyl group of a polyol, forming a urethane linkage between two isocyanate molecules (The University of York, 2013).
Spray Foam Health & Safety
Is spray foam safe to use? Cured SPF is safe but SPF as it is being applied is not. Curing process takes about 24 hours to occur after installation (CBC, 2013). During the spraying process, the aerosolized isocyanates released from the spray gun are irritants to the mucous membranes, eyes, gastrointestinal tract and respiratory tract (National Institute for Occupational Safety and Health, 2013). Exposure to aerosolized isocyanates may lead to severe asthma attacks and sensitization to further exposure (National Institute for Occupational Safety and Health, 2013), (World Health Organization, 2000). The use of adequate ventilation and PPEs during installation are critical to health and safety for workers.
Tips for Selecting a Spray Foam Professional
It is important to hire only certified installers, such as those certified by the Canadian Urethane Foam Contractors Association (CUFCA). Improperly installed or oversprayed SPF may not cure properly and can off-gas harmful chemicals and odors. It may also crack, break, or shrink, incorrectly installed, reducing its efficacy.